Abount Bentonite
What is bentonite?
Introduction: What is bentonite?

Raw ore of bentonite
Bentonite is rock that consists mainly of montmorillonite, a clay mineral, and contains impurities such as quartz, feldspar, and other minerals.
It is believed that deposits of bentonite were formed by reaction of volcanic ash accumulated following volcanic eruptions several million years or several hundred million years ago under certain temperature or pressure conditions or with hydrothermal solutions.
Bentonite deposits exist in the U.S., Europe, China, Japan, and other areas around the world.
Montmorillonite, which is the main component of bentonite, has the characteristic property of swelling to many times its original volume when it absorbs water. This property is called a swelling property. It becomes viscous when dispersed in water, and has the ability to well adsorb various kinds of cations. Thus, it has various properties. Because of these properties, bentonite is used in a wide range of industrial fields. For example, it is used in the fields of castings, civil engineering and construction, toilet sand for pets, chemical products, and many other products.
Bentonite is listed in the Japanese Pharmacopoeia and the Food Sanitation Act, and is also used in cosmetics, pharmaceuticals, or food additives. This is why bentonite is known as clay with 1,000 uses. Recently, it has been studied as a candidate barrier material used in geological disposal of radioactive waste, widening the range of its use.
What is montmorillonite?
The properties of bentonite are decided by the properties of montmorillonite, which is its main component. Therefore, in order to know bentonite, it is important to know the crystal structure and physical properties of montmorillonite first.
Crystal structure
A unit crystal of montmorillonite is a very thin (flat) plate that is about 1 nm thick and about 100 to 1,000 nm wide.
Actually, several unit crystals in this thin plate form are stacked into a mineral particle.
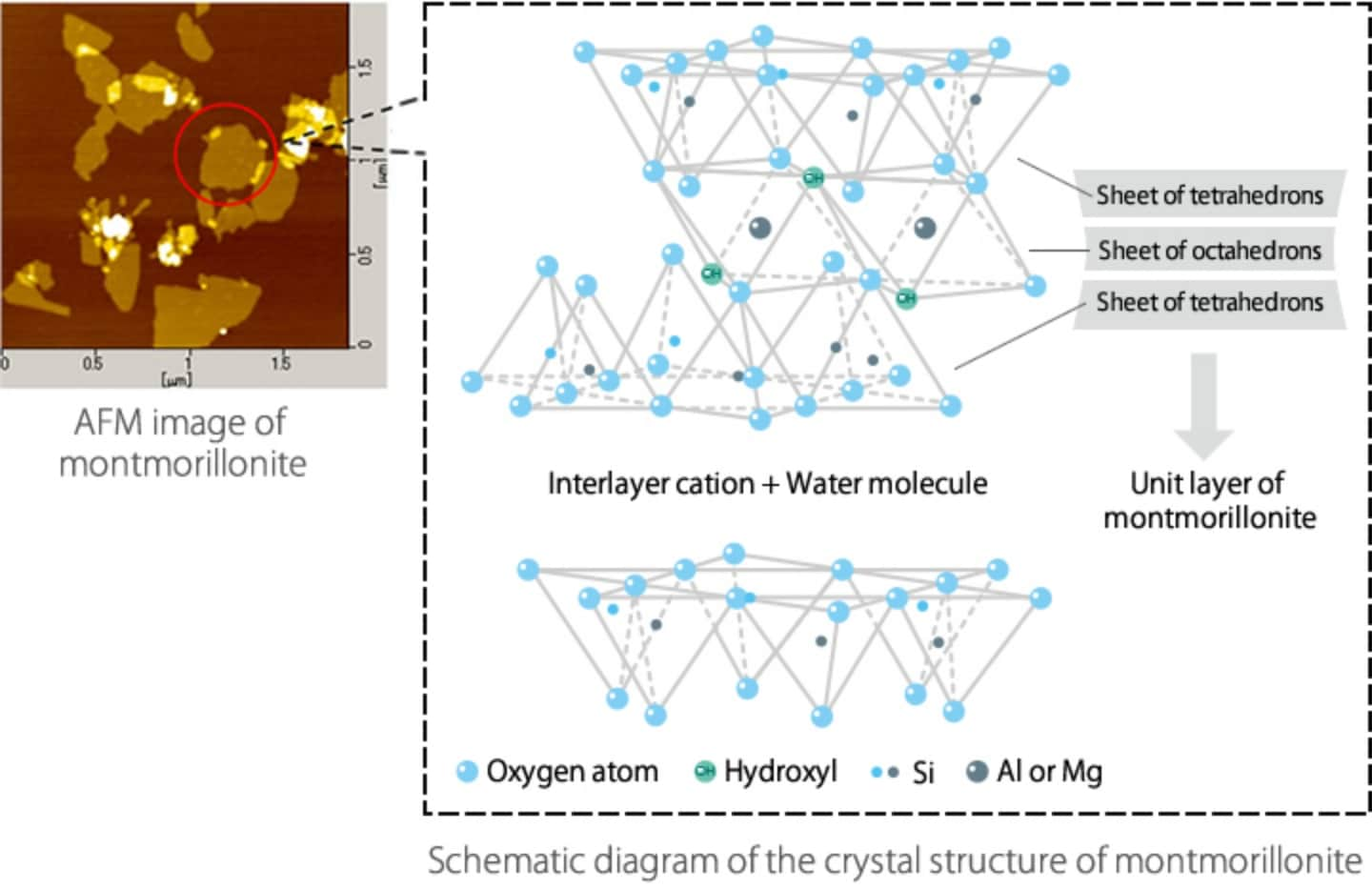
A unit crystal of montmorillonite consists of two sheets made of tetrahedrons of Si (silicon) and O (oxygen), and one sheet made of octahedrons of Al (aluminum) and OH (hydroxyl), which is sandwiched between the two sheets of tetrahedrons. This is called a silicate layer (unit layer). Actually, however, trivalent Al cations in this octahedron sheet are replaced with partly bivalent Mg (magnesium) cations. This is called isomorphous replacement. As a result, the surface of a unit layer is short of electric charge and negatively charged. In order to maintain balance in electric charge, positively charged ions are introduced between unit layers. They are called interlayer cations.
Interlayer cation
There are mainly four types of interlayer cations present in natural montmorillonite: Na+, Ca2+, K+, and Mg2+. Generally, bentonite is roughly classified by the types of interlayer cations in montmorillonite into two types: Na-type bentonite and Ca-type bentonite.
Na-type bentonite
Bentonite that contains many Na+ ions as interlayer cations in montmorillonite is called Na-type bentonite. Na-type bentonite has excellent swelling and thickening properties and suspension stability.
Ca-type bentonite
Bentonite that contains many Ca2+ ions as interlayer cations in montmorillonite is called Ca-type bentonite. Ca-type bentonite is inferior in swelling and thickening properties and suspension stability to Na-type bentonite, but has an excellent water-absorption effect. Artificial Na-type bentonite made by adding a small weight percent of sodium carbonate to Ca-type bentonite is called activated bentonite. Activated bentonite is similar in properties to Na-type bentonite.
Properties of bentonite
Swelling property
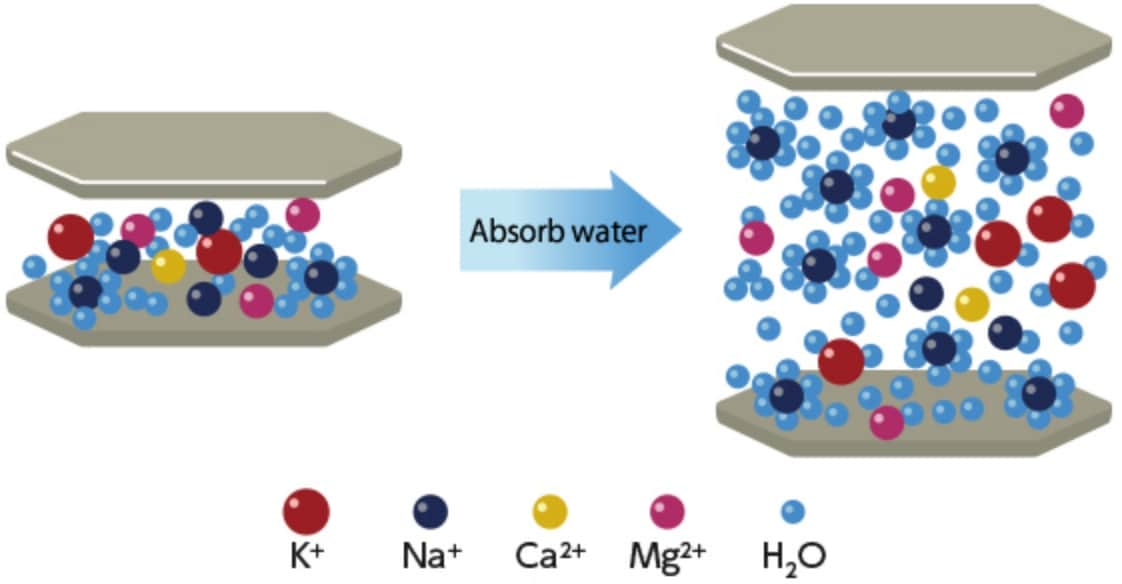
Schematic diagram of swelling of montmorillonite
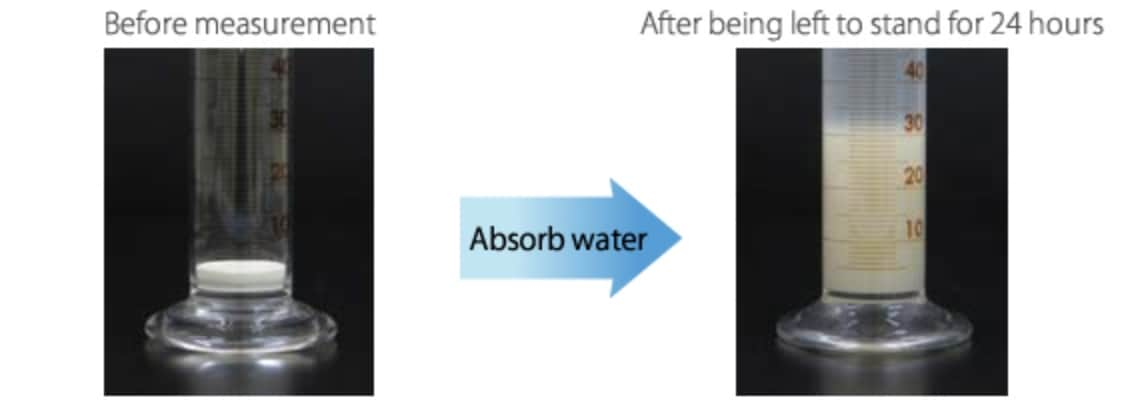
Swelling of bentonite
When montmorillonite comes in contact with water, interlayer cations hydrate with water molecules, increasing the distance between unit layers. This mechanism works differently depending on the types of interlayer cations in montmorillonite. When montmorillonite that contains many Na+ ions as interlayer cations is dispersed in water, Na+ ions hydrate with water molecules, and it is separated into unit layers in the end. This is because the electrical attraction between unit layers by Na+ ions is weak. When this phenomenon occurs, the distance between unit layers is increased to 4 nm or larger, and the clay goes into a state called osmotic swelling.
When montmorillonite that contains many Ca2+ ions as interlayer cations is dispersed in water, Ca2+ ions hydrate with water molecules, but it is not separated into unit layers, and the layers remain stacked. This is because the electrical attraction between unit layers by Ca2+ ions is strong. When this phenomenon occurs, the clay goes into a state called crystalline swelling in which the distance between unit layers remains smaller than 4 nm.
Thickening effect
When montmorillonite that contains many Na+ ions as interlayer cations is dispersed in water, Na+ ions hydrate with water molecules, and it is separated into unit layers. In the unit layer surface of montmorillonite separated into unit layers, a thick hydrate layer is formed, which increases the solid phase rate in the dispersion liquid. This makes the viscosity of the dispersion liquid much higher than that of the dispersion medium or water. This is why the clay shows a viscous property.
Cation exchange
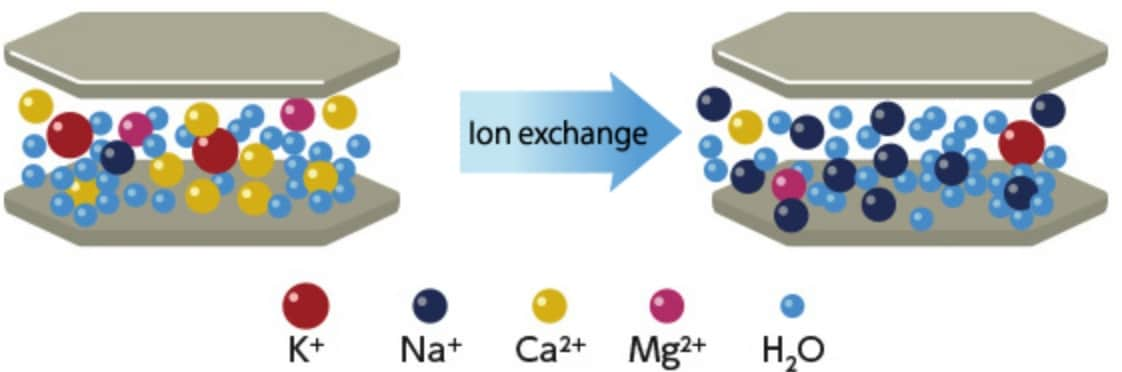
Schematic diagram ofcation exchange of montmorillonite
Interlayer cations in montmorillonite can be exchanged easily with other cations, and are also called exchangeable cations. Treatment methods using activated bentonite are good examples of applying the cation exchange of montmorillonite. Interlayer cations in montmorillonite can be exchanged with not only inorganic cations but also quaternary ammonium ions and other organic cations, and such bentonite is called organic bentonite. Unlike standard bentonite, organic bentonite is difficult to disperse in water, but has an affinity for hydrocarbons and other organic solvents.
Formation of bentonite deposits
Bentonite deposits can be seen in the U.S., Japan, China, Greece, Turkey, and other areas around the world. In Japan, they can be seen in Yamagata, Miyagi, Niigata, Gunma, Aomori, Shimane, Okayama, and other areas. It is thought that bentonite deposits are formed by two major actions: diagenetic alteration and hydrothermal alteration. The KUNIMINE GROUP believes that deposits mined in Yamagata and those in Miyagi and Niigata have been formed by diagenetic alteration and hydrothermal alteration, respectively.
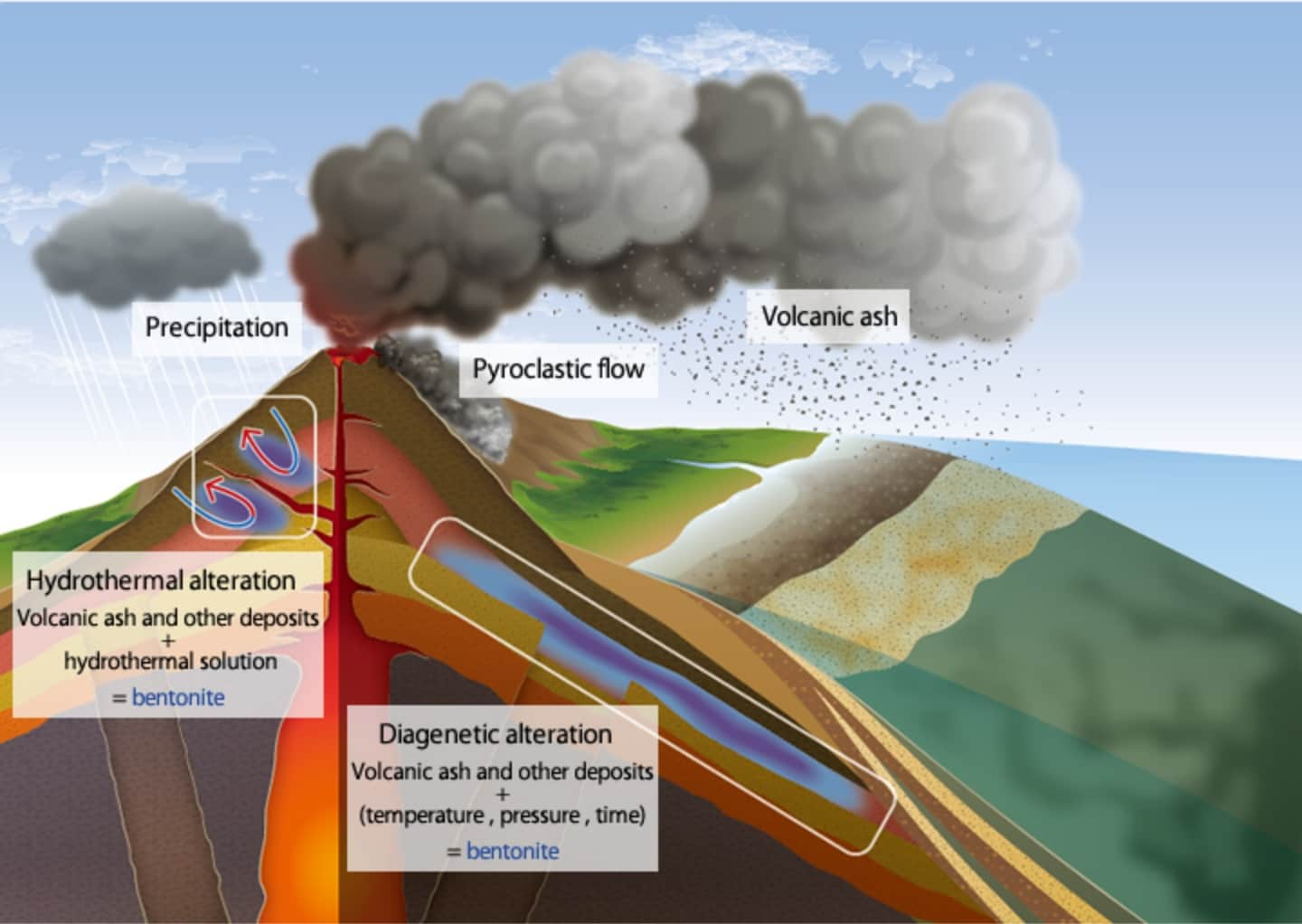
Schematic diagram of formation of bentonite deposits
Diagenetic alteration
Volcanic ash spewed by volcanic eruptions is deposited in the bottom of the sea, lake or other body of water, and buried deep under the ground by crustal movements over time. In the process of the deposit being buried, temperature and pressure conditions and the chemical properties of pore water in it change, forming a new stable mineral. This series of actions is called diagenetic alteration.
Hydrothermal alteration
Volcanic ash and other deposits or igneous rocks react with groundwater heated by underground magma, causing changes in the chemical composition, constituent minerals, or texture of the rocks. This action is called hydrothermal alteration.
Methods for mining bentonite
Bentonite is legally considered as rocks and subject to the Quarrying Act. Ores including bentonite are extracted from mines mainly in two different ways: underground mining and open-pit mining. The KUNIMINE GROUP uses the two different mining methods depending on the way bentonite deposits are present. Specifically, we use underground mining in mines in Yamagata and open-pit mining in mines in Miyagi and Niigata.
Underground mining
This mining method is used to extract mineral deposits present deep in the ground. It has the advantage that quality raw ores mixed with few undesired rocks can be mined without spoiling the natural landscape.

Entrance of a mine where underground mining takes place

Underground mining
Open-pit mining
This mining method is used to extract mineral deposits present near the surface of the ground. It has the advantage that it is highly safe and the mining efficiency is high.

Panoramic view of a mine where open-pit mining takes place

Open-pit mining
Rare stones present in bentonite deposits
During mining of bentonite, carbonate nodules or pyrites are often observed.

Carbonate nodule

Pyrite